TSS assembly pipeline for Rn_EPDnew_001
Introduction
This document provides a technical description of the transcription start site assembly pipeline that was used to generate EPDnew version 001 for R. norvegicus.
Source Data
Promoter collection:
| Name | Genome Assembly | Promoters | Genes | PMID | Access data | ||
|---|---|---|---|---|---|---|---|
| ENSEMBL92 genes | Jul 2014 Rnor_6.0/rn6 | 28847 | 21919 | 29155950 | SOURCE | DOC | DATA |
Experimental data:
| Name | Type | Samples | Tags | PMID | Access data | ||
|---|---|---|---|---|---|---|---|
| lizio17 | CAGE | 13 | 311,502,073 | 29182598 | SOURCE | DOC | DATA |
Assembly pipeline overview
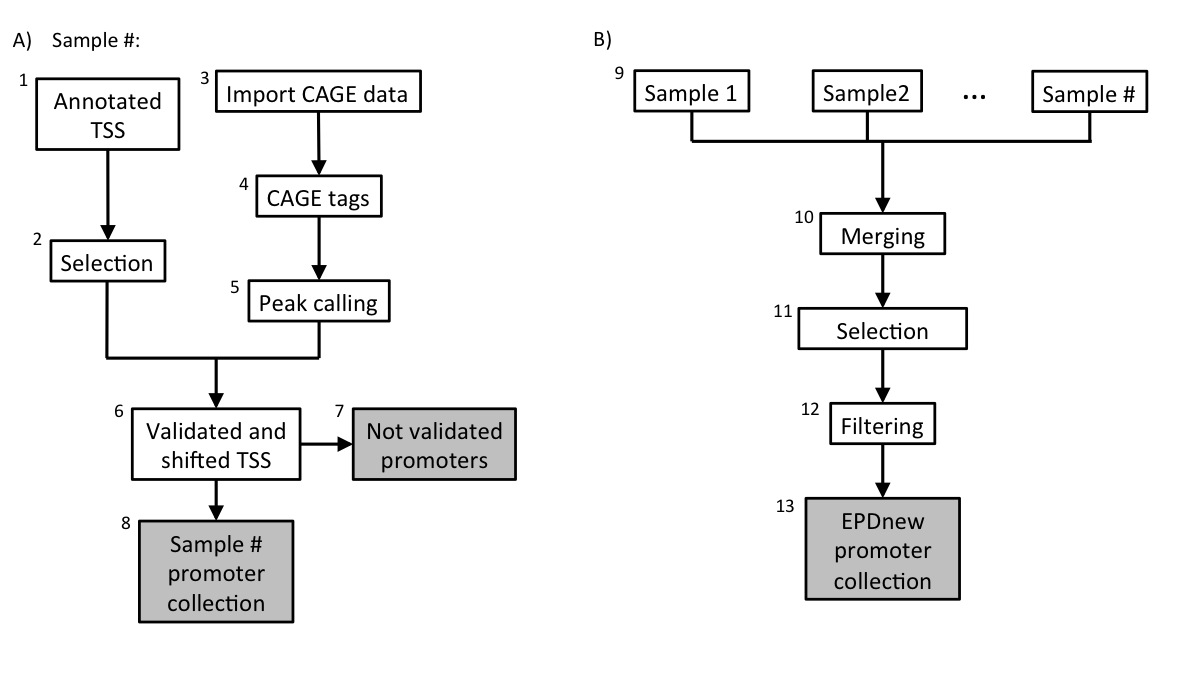
|
Description of procedures and intermediate data files
1. UCSC Download
Data was downloaded from UCSC table browser in June 2018 Then, transcrips have been filtered according to the following rules:- Transcripts of protein coding genes only
- Transcript lies on full chromosomes
- Genes must be annotated [Associated Gene Name present]
- Gene and transcripts status known
Transcripts with the same TSS position were merged under a common ID. As a conseguence, from the 30190 transcripts originally present in the database, 28847 uniquely mapped promoters remained in the input list, covering 21919 genes.
2. UCSC TSS collection
The UCSC TSS collection is stored as a tab-deliminated text file conforming to the SGA format under the name:-
Rn_ucscTSS_rn6.sga
- NCBI/RefSeq chromosome id
- "TSS"
- position
- strand ("+" or "-")
- "1"
- ENSEMBLGeneID .. geneName
3. Data import from FANTOM5
BAM files for high quality CAGE samples (hCAGE) were downloaded from FANTOM5 ftp-site (see link above). Files were then converted into SGA format using in-house software. There are a total number of 13 samples in this collection. Individual SGA files can be downloaded from our ftp website (link above).5. mRNA 5' tags peak calling
For each individual sample (13), peak calling for the merged file has been carried out using ChIP-Peak on-line tool with the following parameters:- Window width = 1
- Vicinity range = 200
- Peak refine = Y
- Count cutoff = 9999999
- Threshold = 5
6. TSS validation and shifting
Each sample in the collection (mRNA peaks and UCSC TSS) was then separately processed in a pipeline aiming at validating transcription start sites with mRNA peaks. A UCSC TSS was experimentally confirmed if an mRNA peak lied in a window of 200 bp around it or it mapped in the 5-UTR region. The validated TSS was then shifted to the nearest base with the higher tag density.8. Promoter collection for each sample
Each sample in the dataset was used to generate a separate promoter collection. Potentially, the same transcript could be validated by multiple samples and it could have different start sites in different samples. To avoid redundancy, the individual collections were used as input for an additional step in the analysis (Assembly pipeline part B).9. Merging collections and second TSS selection
The 340 promoter collections were merged into a unique file and further analysed. The promoter of a transcript was mantained in the list only if validated by at least two samples. Transcript validated by multiple samples could potentially have the TSS set on a broader region and not to single position. To avoid such inconsistency, for each transcript we selected the position that was validated by the larger number of samples as the true TSS.10. Filtering
Transcription Start Sites that mapped closed to other TSS that belonged to the same gene (500 bp window) were merged into a unique promoter following the same rule: the promoter that was validated by the higher number of samples was kept.10. Final EPDnew collection
The 12601 experimentally validated promoters were stored in the EPDnew database, which can be downloaded from our ftp site. Scientists are welcome to use our other tools ChIP-Seq (for correlation analysis) and SSA (for motif analysis around promoters) to analyze the EPDnew database.Last update October 2019